Our Cholesterol Degrading Platform by Repair Biotechnologies
The Cholesterol Degrading Platform
A basis for therapies that are capable of rapidly reversing atherosclerosis and plaque build up, rather than merely slowing down its progression. A basis for therapies for many other important medical conditions driven by the presence of excess cholesterol.
Human cells cannot break down cholesterol. This leads to the abnormal accumulation of excess cholesterol in locations throughout the body and brain. This in turn contributes to the onset and progression of a number of important age-related and obesity-related conditions, including atherosclerosis in the arteries and metabolic dysfunction-associated steatohepatitis (MASH) in the liver. Our Cholesterol Degrading Platform (CDP) therapy addresses this problem of cholesterol accumulation by providing targeted cells with the ability to safely degrade only the unwanted excess cholesterol.
The problem of plaque.
Atherosclerosis is the build up of cholesterol-based plaques that eventually obstruct arteries and block blood flow. This is the most common contribution to heart disease, stroke, and death. Plaque burden is a reliable predictor of cardiovascular events and poor outcomes.
To date no therapy, or even intensive combination of therapies, can reliably and rapidly reduce plaque size and overall plaque burden to a sizable degree in more than a small fraction of treated patients. The few impressive results do not replicate in the broader population.
Our value to patients.
CDP doesn’t merely slow disease progression and the growth of plaque. It reverses it.
Our extensive in vivo data obtained in mouse models of atherosclerosis supports the following hypothesis: by safely breaking down excess cholesterol, therapies based on the Cholesterol Degrading Platform (CDP) can repair the failure of reverse cholesterol transport that is at the heart of atherogenesis. Rather than accumulating to make macrophages dysfunctional and plaques grow in size, excess cholesterol will instead be catabolized.
Currently, medical treatments for atherosclerosis alter aspects of normal lipoprotein metabolism to slow the accumulation of lipoproteins (principally low-density lipoproteins, LDLs) in the vasculature. A large number of clinical trials indicate that statins, which are inhibitors of cholesterol production, reduce cardiovascular events (e.g. heart attacks and strokes) by about 25%. While this represents a remarkable achievement, a 25% reduction means that 75% of patients with cardiovascular disease will still suffer potentially life-ending cardiovascular events.
Shrinking of existing atherosclerotic plaque burden is strongly correlated with reduced incidence of cardiovascular events. Statin treatment does not usually significantly reduce plaque, and even in the cases where it can, statins must be aggressively administered to achieve LDL <70 mg/dL and HDL >60mg/dL. Such regimens come with increased risk of weight gain, worsening of diabetes, muscle aches and pain, kidney and liver complications, and brain hemorrhage. Furthermore, pregnant women cannot use statins because of the known deleterious effects on fetal development. Finally, the average degree of shrinkage of established plaque is modest at best, and in fact close to zero on average as assessed in meta-analyses of large clinical trials.
Current therapies thus largely act to slow the progression of existing atheromas only, and these structures remain within the walls of affected vascular structures to cause potentially lethal complications, even after lengthy periods of treatment.
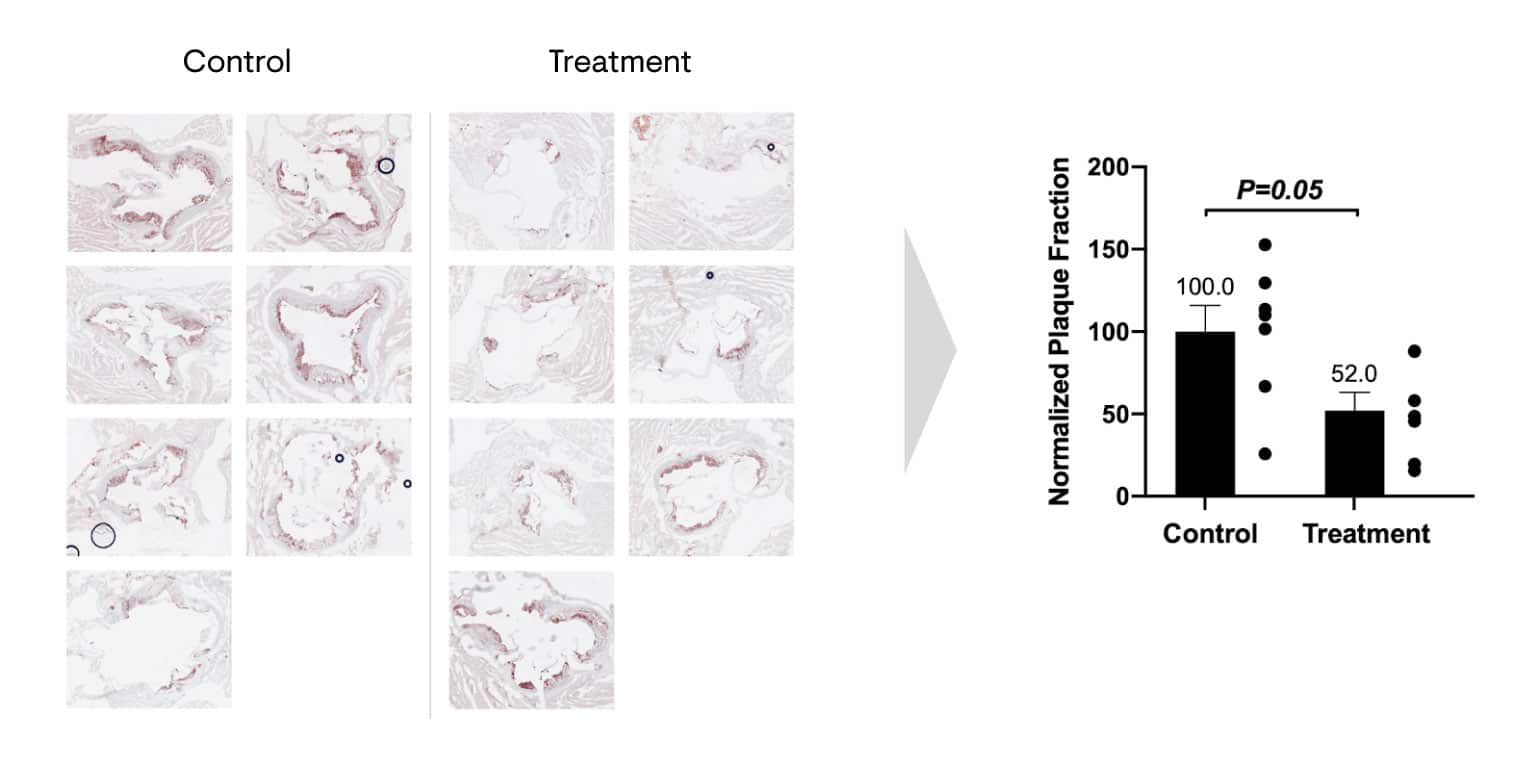
In contrast, a single treatment of Repair Biotechnologies’ early, unoptimized CDP therapy, delivered as an adeno-associated virus (AAV) gene therapy vector, reduced atherosclerotic plaque lipids by 48% in mice. The image shows stained sections of the aortic root from the control and treatment groups, in which areas of plaque show up in red. In this experiment, atherosclerosis-prone mice were placed on a high-fat diet and angiotensin II for 4 weeks. The treatment group was administered CDP therapy, and at 4 weeks post-injections, mice were euthanized.
Various organs including the heart and aortic tree were removed and processed for histology. Throughout the experimental protocol, mice in all groups were mobile, looked healthy and did not display any weight loss or signs of distress. Liver AST and ALT levels and their ratios remained unchanged between control-treated and CDP-treated groups.
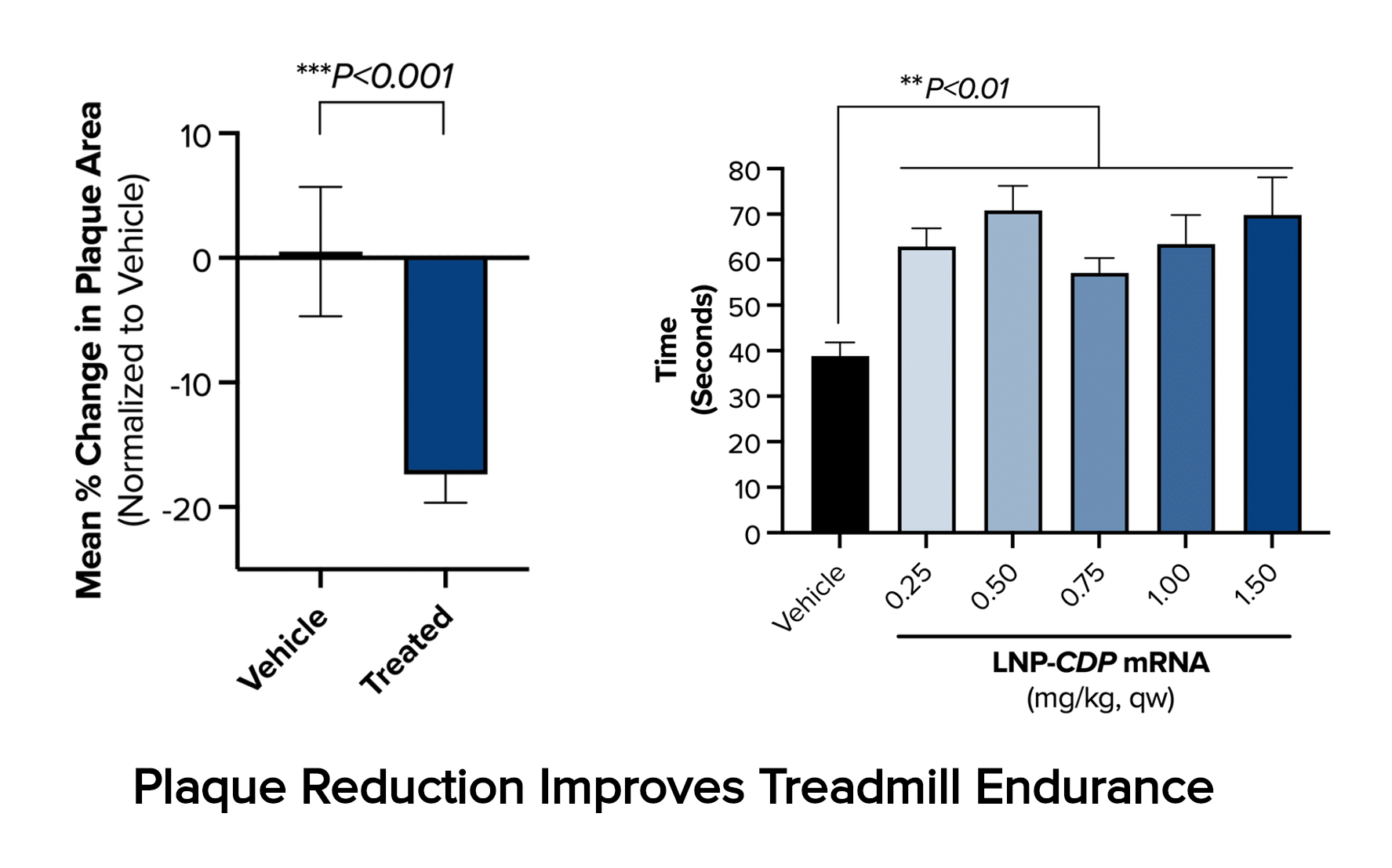
The present optimized form of CDP therapy moving into clinical development makes use of lipid nanoparticle (LNP) delivery of messenger RNA (mRNA). In the LDLR-null mouse model of homozygous familial hypercholesterolemia, a rare condition exhibiting greatly accelerated development of atherosclerosis, six weeks of treatment with this LNP-mRNA CDP therapy produces a 17% reduction in aortic plaque cross-sectional area and consequent sizable improvement in cardiovascular function, as illustrated by improved treadmill performance.
Unlike current standards of care such as statins and the more modern PCSK9 inhibitors, both of which aim to reduce circulating levels of LDL-cholesterol, and have a minimal impact on atherosclerotic plaque size and burden, treatment with CDP therapies has resulted in a sizable reduction in plaque burden in the aortic roots of atherosclerosis-prone mice.